Radiocarbon dating is a type of radiometric dating technique that is used to determine the age of prehistoric fossils, bones, organic materials in rocks, and pretty much everything that has carbon in it. This dating method is based on the properties of carbon-14, a radioactive isotope of carbon.
Isotopes of carbon
Carbon has fifteen known isotopes. Three of them are naturally occurring. Carbon-12 is the most abundantly occurring isotope with a share of approximately 99% of all carbon on Earth. Carbon-13 makes up about approximately 1% and carbon-14 (also known as radiocarbon) exists only in trace amounts.
Carbon-12 and carbon-13 are stable. But, carbon-14 is unstable and has a half-life of 5,730 ± 40 years. Half-life is the time required for a radioactive sample to decay from its initial amount to exactly half its size. As carbon-14 is unstable, it constantly decays into the stable nitrogen-14.
Carbon-14 cycle
All lifeforms are made up of carbon. Approximately 18% of our human body is made up of carbon. But, as mentioned earlier, most of this carbon is carbon-12.
Carbon-14 is very rare. But, the ratio of carbon-12 to carbon-14 is the same in all living things and in the atmosphere of that particular time period. So, how do these carbon-14 atoms enter into living organisms?
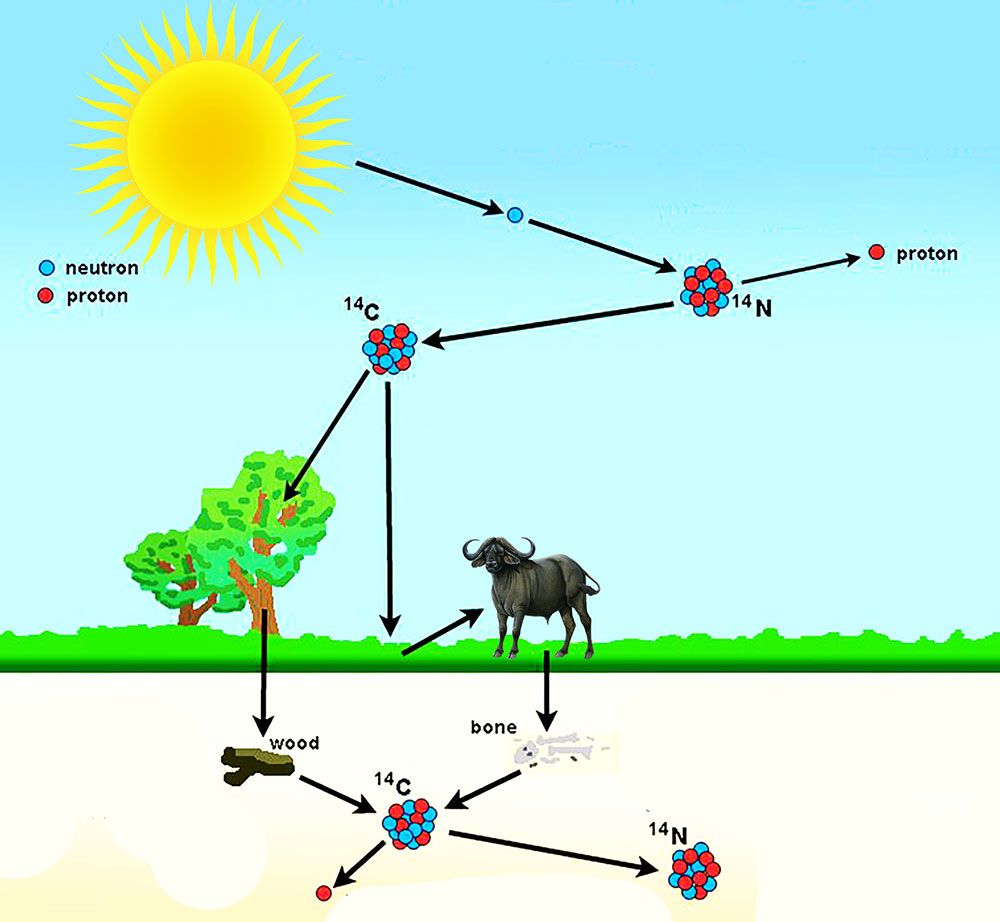
Earth’s upper atmosphere is constantly bombarded by the cosmic rays from the outer space resulting in the formation of lighter particles like x-rays, muons, protons, alpha particles, pions, electrons, and neutrons. These neutrons interact with the nitrogen-14 atoms in our atmosphere. And as a result, nitrogen-14 decays into carbon-14.
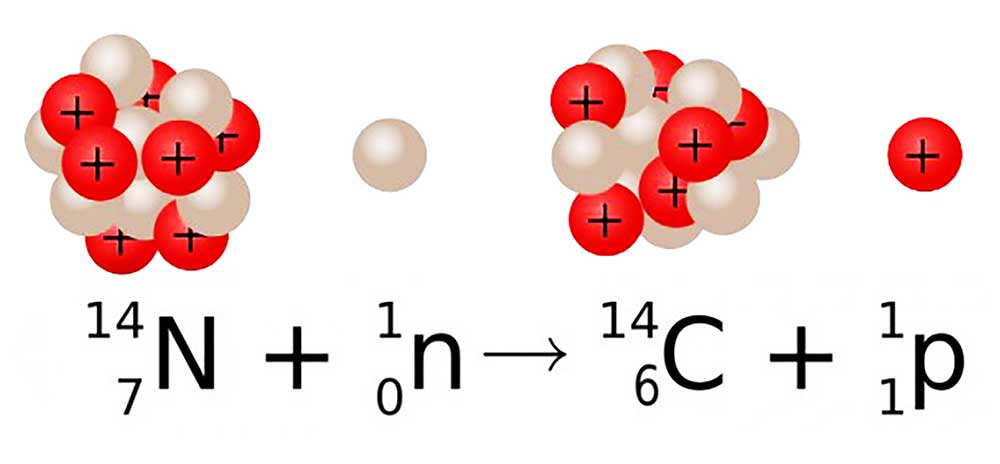
Carbon-14 enters plants via photosynthesis. And, as animals consume these plants, carbon-14 enters the food cycle. Thus, it ends up in all living organisms. As long as the organism is surviving, the ratio of carbon-12 to carbon-14 in them is the same as the atmospheric ratio in that time period.
How radiocarbon dating is done on fossils?
But, when a living organism dies, the number of carbon-14 atoms in them starts to decrease as it decays back into nitrogen-14. But, carbon-12 is stable, so it remains constant.
As a result of this beta decay, the ratio of carbon-12 to carbon-14 in them starts changing from that of the atmospheric ratio in their time period. And, this difference in the ratio is what we need in order to calculate the age of an organic sample.
Accelerator mass spectrometer
Accelerator mass spectrometry or AMS is a technique that is being used today to analyze the concentration of carbon-14 atoms in organic samples for radiocarbon dating.

An accelerator mass spectrometer, as the name suggests, it accelerates negatively charged ions to high energies in order to separate the rare carbon-14 atoms from the abundant neighboring carbon-12 atoms for mass analysis.
Carbon dating can be used to date samples that are up to 50,000-60,000 years old. But, with a half-life of 5730 ± 40 years, only about 3% of the original carbon-14 remains in a sample after 30,000 years.
Beyond 40,000-50,000 years, it becomes challenging to measure carbon-14 with conventional laboratory methods. Another challenge is the dilution of radiocarbon in the atmosphere due to man-made carbon emissions from industries and nuclear testing. So, an updated database of carbon-12 to carbon-14 ratio is used by scientists to consider humans’ effects on the atmosphere while dating samples.
Development of radiocarbon dating

The presence of radiocarbon in our atmosphere was first theoretically suggested by Franz Kurie in 1934. Carbon-14 was discovered on February 27, 1940, by Martin Kamen and Sam Ruben at the University of California Radiation Laboratory in Berkeley, California.
In 1946, Willard Frank Libby, a professor at the University of Chicago, proposed an innovative method to date organic samples using the properties of carbon-14. Since then, radiocarbon dating has revolutionized the fields of archeology and geology. Willard Frank Libby was awarded the Nobel prize for Chemistry in 1960 for his work on radiocarbon dating.